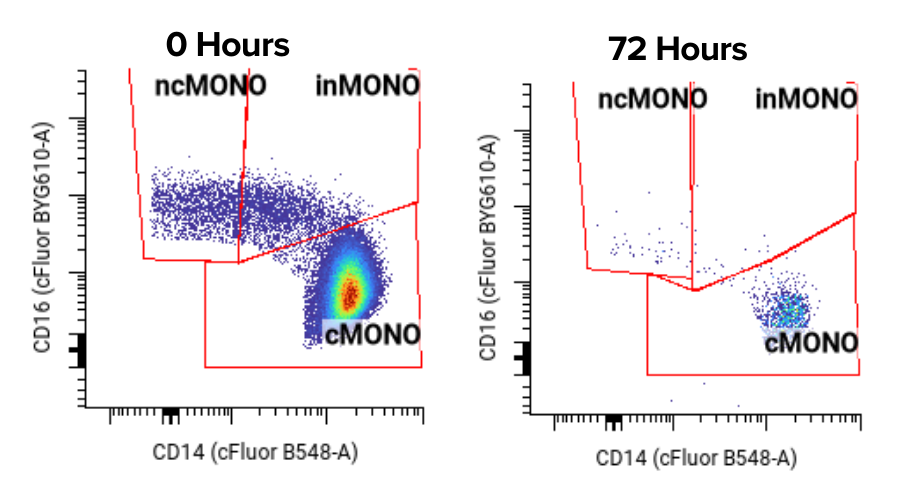
Translational scientists are used to taking a fine-tooth comb through costs with CROs. Data pull fees, panel verification, validation, reverification fees, setup fees, etc. It all adds up.
But there’s one line item that you should look at that impacts the financials: assay noise. This is measured by "coefficient of variation" (CV). The lower, the better. A CV of 3% is a LOT better than a CV of 20%, as we'll see soon.
When we measure anything, we’re looking to measure clinical truth, not random noise.
Think Ozempic and a weight scale. If your weight scale is "noisy", someone who is going down from 130 lbs to 110 lbs might get reported as going from 130 lbs to 135 lbs! So then, it looks like your drug isn’t working. Or worse, you need a TON more patients to show that your drug is working. Which means money and wasted time.
Let’s see this in a bit more detail:
- Standard Assay (20% CV): You might need 32 patients to prove efficacy.
- High-Precision Assay (3% CV): You might only need 12 patients to show the exact same biological effect, and with much cleaner separation.
At $75K per patient, that’s $900K wasted.
That difference isn't just data could represent millions of dollars in recruitment fees, extended site monitoring, and delayed time-to-market.
We’ve released a free calculator at precision.teiko-labs.com. You can set parameters like the effect size of your drug, estimated cost, the number of patients, and more. Let us know what you think!
.png)